Introduction:
Risk stratification approaches using clinical criteria or biomarkers in acute graft-versus-host disease (GVHD) have traditionally been implemented at diagnosis or initiation systemic treatment with corticosteroids. For patients who require second-line treatment, the significance of biomarkers at salvage therapy and the relationship between biomarker risk and choice of therapy are unknown.
Methods:
We studied adult patients who received second-line treatment for acute GVHD between January 2016 and December 2021 in the Mount Sinai Acute GVHD Consortium (MAGIC) database, which collects clinical data and longitudinal serum samples from allogeneic HCT recipients using a rigorous PRoBE study design. Exclusion criteria included relapse of underlying malignant disease prior to second-line therapy, missing response data, or missing serum sample. Serum samples collected from 7 days prior to 3 days after initiation of second-line therapy met criteria for biomarker analysis. The MAGIC algorithm probability (MAP) was calculated as a single value based on concentrations of ST2 and REG3α and was stratified at a threshold of 0.290 to define high MAP and low MAP groups. The primary objective evaluated the association between MAP and survival for patients receiving second-line therapy for acute GVHD. The key secondary objective was to investigate the MAP and choice of second-line systemic agent on Day 28 overall response rate (ORR) and survival.
Results:
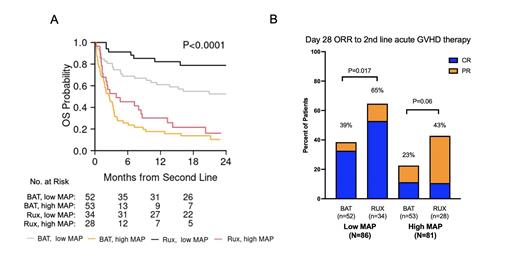
In total, 316 adult patients received second-line treatment for acute GVHD; 149 patients (47%) were excluded for disease relapse (n=27), missing response data (n=48), or missing sample (n=74).Of the 167 evaluable patients, most had steroid-refractory acute GVHD (n=146, 87%), rather than steroid-dependent (n=8, 5%) or steroid-sensitive (n=14, 8%) disease. 62 patients received ruxolitinib while 102 received other systemic agents (best available therapy: BAT). Baseline clinical characteristics of the study population according to treatment choice were similar, except for donor type (Rux: MRD 15%, MUD 74%, haplo 11%; BAT: MRD 30%, MUD 67%, haplo 3%; P=0.014) and median days from corticosteroids to second-line treatment (Rux: 18, BAT: 12; P=0.02).In total, 81 patients had high MAPs and 86 patients had low MAPs, with similar distribution in each treatment cohort (P=0.53). For the entire cohort, the median follow-up for survivors was 22 months (range, 1.5-27).The MAP separated patients into 2 groups (high vs low) with significantly different mortality (NRM at 2 years, 76% vs 29%, P<.0001) and survival (OS at 2 years, 12% vs 63%, P<.0001). Patients who received ruxolitinib as second-line therapy had lower mortality (NRM at 2 years, 35% vs 60%, P<.002) and improved survival (OS at 2 years, 51% vs 32%, P<.008), compared to those who received other systemic agents. However, the outcomes of patients with high MAP were poor, regardless of second-line treatment choice ( Figure 1A). The MAP (low vs high), time from corticosteroids to second-line treatment (≥14 days vs < 14 days), GVHD grade at second-line (0-2 vs 3-4), and choice of second-line therapy (Rux vs BAT) were all significant (P<0.05) predictors of lower NRM and higher OS in multivariate analysis. The MAP also separated patients into 2 groups with significantly different D28 ORR (low: 49%, high: 30%, P<.0001). In agreement with prospective trials, ruxolitinib resulted in higher D28 response rates compared to other systemic therapies (ORR 54.9% vs 30.5%, P=0.005), which was observed in both low MAP (ORR, 64.5% vs 38.5%, P=0.017) and high MAP cohorts (ORR, 42.9% vs 22.6%, P=0.06) ( Figure 1B). The MAP (low vs high), GVHD grade at second line (0-2 vs 3-4), and choice of second-line therapy (Rux vs BAT) were significant predictors (P<0.05) of higher D28 ORR in multivariate analysis.
Conclusion:
The MAP measured at the initiation of second-line systemic treatment for acute GVHD predicts NRM and OS, as well as treatment response. The outcomes of patients with high MAP are poor, regardless of second-line treatment choice, and the higher CR rate in low MAP patients may drive higher survival rates. Incorporation of MAP into clinical trials in this setting warrants investigation. The results also support the current use of ruxolitinib as second-line treatment for acute GVHD, particularly in patients with low MAP.
Disclosures
Defilipp:Inhibrx: Consultancy; Incyte: Consultancy, Research Funding; Regimmune: Research Funding; Taiho Oncology: Research Funding; Sanofi: Consultancy; PharmaBiome AG: Consultancy; Ono Pharmaceutical: Consultancy; MorphoSys: Consultancy. Choe:Opna: Other: Receipt of equipment, materials, drugs to institution, Research Funding; Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Receipt of equipment, materials, drugs to institution; MJH Life Sciences: Honoraria; Actinium Pharmaceuticals: Other: Support for attending meetings and/or travel; NIH National Cancer Institute: Research Funding. Grupp:Cabaletta: Consultancy, Membership on an entity's Board of Directors or advisory committees; Allogene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Juno: Consultancy, Membership on an entity's Board of Directors or advisory committees; Cellectis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Adaptimmune: Consultancy, Membership on an entity's Board of Directors or advisory committees; CBMG: Consultancy, Membership on an entity's Board of Directors or advisory committees; Servier: Research Funding; Kite: Research Funding; Jazz: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Vertex: Consultancy, Research Funding; Novartis: Consultancy, Research Funding. Kitko:Horizon: Membership on an entity's Board of Directors or advisory committees. Qayed:Vertex: Honoraria; Novartis: Honoraria. Reshef:Allogene: Consultancy; Bayer: Consultancy; Precision Biosciences: Research Funding; J&J: Research Funding; Jasper: Consultancy; Capstan: Consultancy; MidaTech: Consultancy; Orca: Consultancy; Synthekine: Consultancy, Research Funding; CareDx: Research Funding; TScan: Consultancy, Research Funding; Incyte: Consultancy, Research Funding; Instil Bio: Consultancy; Regeneron: Consultancy; Takeda: Consultancy, Honoraria, Research Funding; Atara Biotherapeutics: Consultancy, Research Funding; Bristol Myers Squibb: Honoraria, Research Funding; Gilead Sciences: Consultancy, Honoraria, Other: Travel support, Research Funding; Quell Biotherapeutics: Consultancy; Sanofi: Research Funding; Immatics: Research Funding; TCR2: Research Funding. Zeiser:incyte: Consultancy, Honoraria; novartis: Consultancy, Honoraria, Research Funding; MNK: Consultancy, Honoraria; Medac: Honoraria; Sanofi: Consultancy, Honoraria; VectivBio: Consultancy. Ferrara:Viracor: Patents & Royalties: GVHD biomarker patent.. Nakamura:Leukemia & Lymphoma Society: Other: grant reviewer; NCCN: Other: guideline panel for HCT; International Consortium: Other: consortium chair; Sanofi: Consultancy; Napajen: Consultancy; Blue Bird: Consultancy; Mt. Sinai: Other: Acute GVHD; NCTN Lymphoma Steering Committee: Membership on an entity's Board of Directors or advisory committees; BMT CTN Steering Committee: Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Consultancy, Other: research collaboration; Miyarisan: Research Funding; Omeros: Consultancy. Levine:Kamada: Consultancy; Mesoblast: Consultancy; Sanofi: Consultancy; X4 Pharmaceuticals: Consultancy; Viracor: Patents & Royalties: GVHD biomarker patent.; Genentech: Research Funding; Mesoblast: Research Funding; Incyte: Research Funding; Inhibrx: Consultancy; Incyte: Consultancy; Editas: Consultancy; Equillium: Consultancy; Bluebird Bio: Consultancy.